The history of plasma fractionation began with the need to develop blood serum products to help soldiers suffering from shock and burns in the Second World War. Dr. Edwin Cohn, of Harvard University, was a key figure in the development of a process to separate proteins from human plasma.

Today, the “Cohn fractionation process,” remains the basis of many plasma fractionation facilities’ manufacturing processes around the world.
Originally, the aim of plasma fractionation was to separate albumin from plasma to treat injured soldiers. Albumin represents 55-60% of the total protein volume of plasma, and is easier to separate from plasma than any other proteins. Over time, a number of additional proteins were separated from plasma and used clinically. Each of these proteins can be used to treat one or several medical conditions, many of which are orphan diseases – meaning the number of patients affected is comparatively small. Many of the diseases treated with proteins derived from plasma are serious, life-threatening conditions.
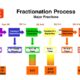
The Cohn fractionation process originally developed in the 1940’s involved modifying the pH, the ethanol concentration and the temperature of the plasma to separate its proteins through precipitation into five “fractions” (I-V).
Before starting the fractionation process, the “cryoprecipitate” which contains coagulation factors VIII and IX, is removed. These coagulation factors are missing in hemophilia patients (factor VIII in hemophilia A, and factor IX in hemophilia B), putting them at risk of uncontrolled bleeding. In the 1960’s, methods were developed to purify factor VIII and later factor IX from fraction I of plasma, enabling hemophilia patients to use these purified proteins in concentrated form for treatment. Before factors VIII and IX concentrates were developed, hemophilia patients had to infuse fresh plasma, or cryoprecipitate in non-concentrated form, or even whole blood to stop bleeding. These modes of hemophilia treatments are still a reality for a few in a some low-income countries.
In the 1950’s intramuscular immunoglobulin (IMIG) was developed from fractions II and III and introduced as replacement therapy for patients with congenital antibody deficiencies. Since the intramuscular injections were painful, only small volumes could be administered, which prevented adequate dosing. In the 1980’s, the purification methods had sufficiently improved to enable the production of “intact” molecules (similar to those found in human plasma) with full biological efficacy. As they were better tolerated, these preparations could be administered intravenously, significantly enhancing their clinical efficacy. This makes it possible for intravenous immunoglobulin (IVIG) to be prescribed for a growing number of medical indications. In the late 1990’s, a new form of administration of immunoglobulin using the subcutaneous route became available (SCIG), offering patients an alternative to the intravenous route.
In the late 1980’s, alpha-1 proteinase inhibitor (A1PI) was purified from fraction IV and commercialized for the treatment of a hereditary form of emphysema caused by low alpha-1 protein levels. The fraction IV of plasma also comprises antithrombin III, another protein introduced in the market in the 1980’s while fraction V contains almost exclusively albumin.
Over the years, the Cohn fractionation process was improved and complemented by new technologies. Using chromatography processes to purify specific proteins, the industry has significantly increased production yields, in particular IVIG. More recently, some companies, such as Prometic and Evolve Biologics are using ligands to extract and purify plasma proteins achieving higher yields but they have not yet introduced any product on the market.
Companies continuously endeavor to maximize their production yields so as to get the most from human plasma and generate more revenues from a liter of plasma.
Today’s plasma fractionation plants are large and complex, capable of fractionating millions of liters of plasma into a wide variety of therapeutic products.

Read the full article: https://marketingresearchbureau.com/the-plasma-industry/